Cancer treatment rarely offers clean victories. Most therapies focus on shrinking tumours or delaying recurrence. Long-term protection remains elusive.
However, new clinical data from Moderna and Merck suggest a meaningful outcome. Their experimental mRNA cancer vaccine, used alongside Keytruda, shows protection lasting up to five years after treatment.
That timeline matters. Five years often marks the psychological and clinical threshold where patients and doctors begin to speak about remission with confidence. While questions remain, this trial hints at something larger. It points toward a future in which cancer care includes personalised immune training, not just aggressive intervention.
What’s Happening & Why This Matters
A Trial Focused on High-Risk Melanoma

The trial targets patients with high-risk melanoma, one of the most aggressive skin cancers. Researchers combine a personalised mRNA vaccine with Keytruda, Merck’s widely used immunotherapy drug. The vaccine trains the immune system to recognise tumour-specific mutations. Each dose uses genetic data from the patient’s own cancer.

That approach differs from traditional vaccines. Instead of preventing infection, it teaches the body to hunt residual cancer cells. Earlier results already showed promise.
Now, the five-year follow-up strengthens that signal.
Five-Year Data Extends Earlier Gains
Earlier trial data tracked patients at the two-year mark. At that stage, the results already stood out. Among 107 patients who received the mRNA cancer vaccine plus Keytruda, 22 per cent experienced recurrence or death.
In contrast, 40 per cent of patients treated with Keytruda alone saw the same outcomes. That translated to a 44 per cent reduction in risk. Fast forward to five years. The companies now report a 49 per cent reduction in recurrence or death.
Notably, this figure matches the three-year results. Consistency matters here. It suggests the benefit does not fade quickly over time.
Side Effects Remain Manageable
Long-term therapies often fail due to tolerability. This trial avoids that pitfall so far. Reported side effects remain similar to earlier analyses. Patients experience fatigue, injection-site pain, and chills most often.
Crucially, adverse events appear comparable between both treatment groups. That parity supports further expansion into late-stage trials. Safety rarely grabs headlines. Still, it determines whether a therapy survives regulatory scrutiny.
Moderna Frames This as a Platform Moment
Moderna leadership emphasises the extensive implications. Kyle Holen, Senior Vice President at Moderna, describes the data as evidence of prolonged benefit. He frames the trial as proof that mRNA technology extends beyond infectious disease.
According to Holen, Moderna now runs eight additional Phase 2 and Phase 3 trials. These target the lung, bladder, kidney, and other cancers. This matters because oncology pipelines often stall after early success. Here, Moderna signals sustained momentum.
Merck Sees Momentum Across Tumour Types
Merck echoes that optimism. Marjorie Green, Senior Vice President at Merck, calls the five-year data a “meaningful milestone.” She also points toward the broader INTerpath clinical program, which spans multiple cancer types.
That framing matters. Merck already anchors its oncology strategy around Keytruda. If personalised mRNA vaccines amplify Keytruda’s effectiveness, the commercial and clinical upside multiplies.
Why Five Years Changes the Conversation
Cancer recurrence often happens within the first few years after treatment. Each year without relapse improves long-term survival odds. Five years is identified as a physiological and psychological turning point.
Patients regain confidence. Clinicians adjust monitoring schedules. Therefore, sustained protection across that window shifts expectations. It reframes cancer care from reactive treatment to immune-based prevention.
The approach reflects how vaccines changed infectious diseases. The difference lies in personalisation.
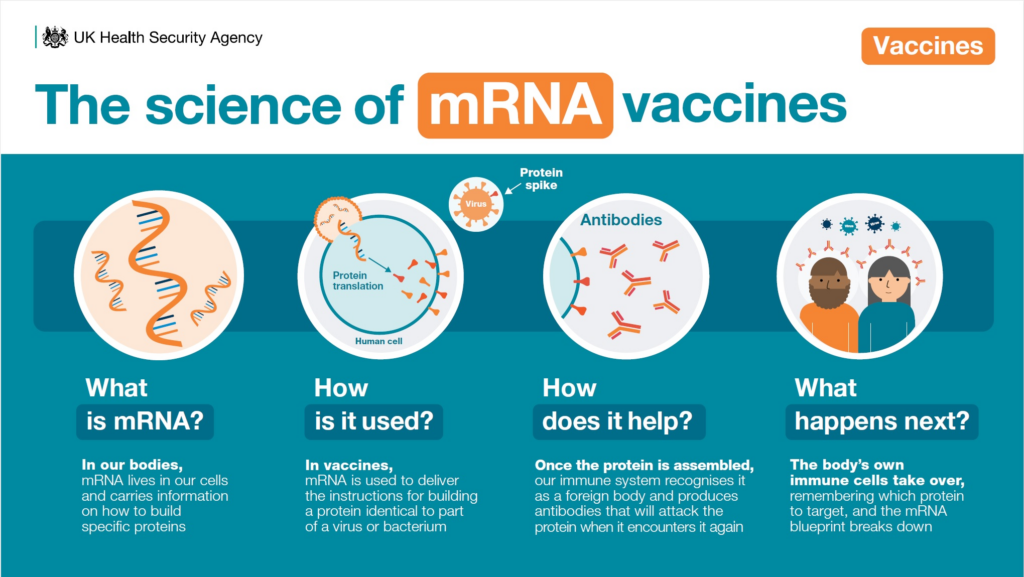
The Science Behind the Promise
mRNA vaccines work by delivering genetic instructions. Cells briefly produce tumour-specific proteins. The immune system learns to recognise those markers. Unlike chemotherapy, this method avoids broad cellular damage.
Instead, it sharpens immune memory. That precision explains why recurrence rates fall without a spike in side effects. Importantly, this vaccine adapts to each tumour. No two patients receive the same formulation.
Caution Still Applies
Despite encouraging results, gaps remain. The companies have not yet published full five-year datasets. Peer review and independent analysis still matter.
Additionally, the trial size remains modest. Larger populations may surface new risks or limitations. Regulators will scrutinise durability, manufacturing scale, and cost.
Political Headwinds Complicate the Picture
The broader environment adds uncertainty. mRNA technology now faces political resistance in the United States. Health Secretary Robert F. Kennedy Jr. continues to criticise mRNA vaccines. In August, federal officials cancelled $500 million in grants tied to mRNA pandemic preparedness.
That decision casts a long shadow over future funding. While cancer vaccines differ from COVID-19 shots, public perception still matters. Policy signals influence investment and trial expansion.
Why This Trial Still Breaks Through
Despite these obstacles, the science speaks clearly. The data shows consistency across years. The immune response holds. Side effects remain stable. More importantly, patients live longer without recurrence. That combination rarely appears this early in new treatment categories.
TF Summary: What’s Next
The Moderna-Merck mRNA cancer vaccine trial marks a quiet turning point. Five-year protection reshapes how clinicians think about post-cancer care. It suggests immune memory, not constant intervention, drives long-term outcomes.
MY FORECAST: Late-stage trials expand across additional cancers within two years. Regulatory approval arrives first in melanoma. If politics does not derail funding, personalised cancer vaccines enter mainstream oncology before the decade ends.
— Text-to-Speech (TTS) provided by gspeech


