Neuralink, the neurotechnology company founded by Elon Musk, is making a bold move north. The company has received Health Canada’s approval to expand its operations and is looking for six patients with quadriplegia to receive its flagship N1 brain implant. This expansion is a leap for Neuralink as it broadens its reach to Canadian patients.
What’s Happening & Why This Matters
Neuralink’s latest venture, named CAN-PRIME, will implant the N1 brain chip in selected Canadian patients. The goal is to help individuals who are unable to use their hands by allowing them to control devices like phones and computers with their thoughts. This initiative closely mirrors Neuralink’s U.S.-based human testing trials, which are exploring similar eligibility criteria and objectives.
The first Canadian trial will be conducted by Toronto’s University Health Network (UHN). Dr. Kevin Smith, President and CEO of UHN, expressed pride in leading this cutting-edge research. He highlighted the role of their medical and research professionals in advancing neurosurgery and providing innovative treatments. UHN’s partnership with the University of Toronto strengthens the research effort, with multiple hospitals involved in the trial, including Toronto General Hospital and Toronto Western Hospital.
How the N1 Brain Chip Works
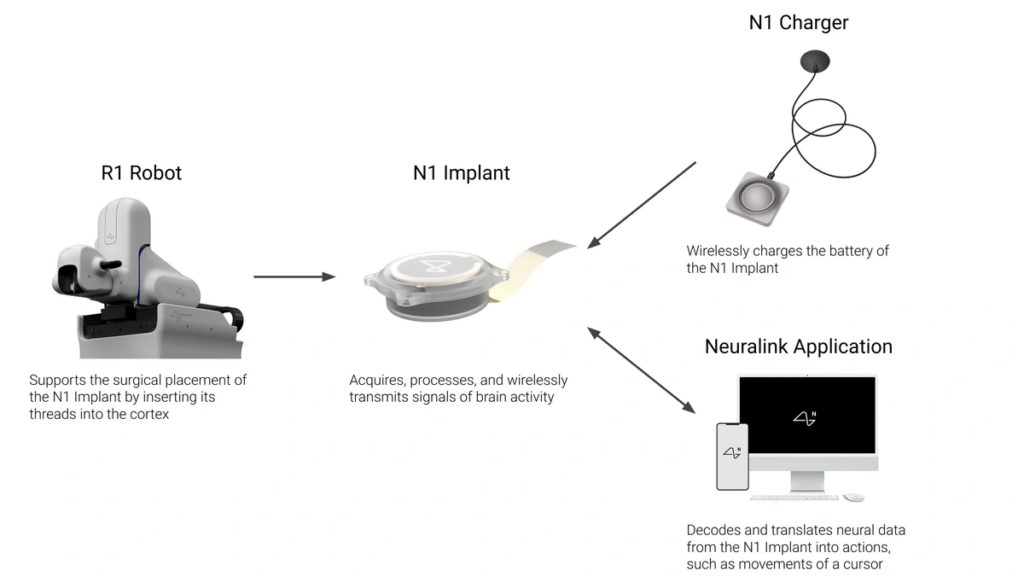
Neuralink’s N1 implant, delivered by its R1 Robot, attaches tiny threads to the patient’s brain. Once implanted, the device is essentially invisible and the threads transmit brain activity, enabling patients to interact with technology in new ways. Although some threads have detached in early trials, the device continues to function effectively.
The first U.S. patient, Noland Arbaugh, is now able to play video games, use a computer, and engage with technology—activities that were once beyond his reach. The chip’s potential goes far beyond gaming, however, offering significant improvements in daily functioning for those with limited motor abilities.
TF Summary: What’s Next
Neuralink’s Canadian expansion is an exciting development for brain-computer interface bio-med. With trials in the U.S., and now Canada, underway, the company is proving its concept in real-world settings. The potential for transforming lives, especially for those with physical limitations, is substantial. As the trials progress, Neuralink may expand into other new territories — bringing cutting-edge technology to more patients in need.
— Text-to-Speech (TTS) provided by gspeech