A Finnish startup company called Sooma has developed a portable brain stimulation device for treating depression. This device uses a treatment called Transcranial Direct Current Stimulation (tDCS). tDCS provides an alternative for people who may not respond well to medications or who have limited access to psychotherapy.
What’s Happening & Why This Matters
tDCS uses a low electrical current to stimulate specific parts of the brain. The mild electrical currents administered through the cap are said to influence the activity of specific brain cells responsible for releasing neurotransmitters related to mood. Recent research has shown that tDCS is linked to increased grey matter in areas of the brain associated with loss of grey matter from depression.
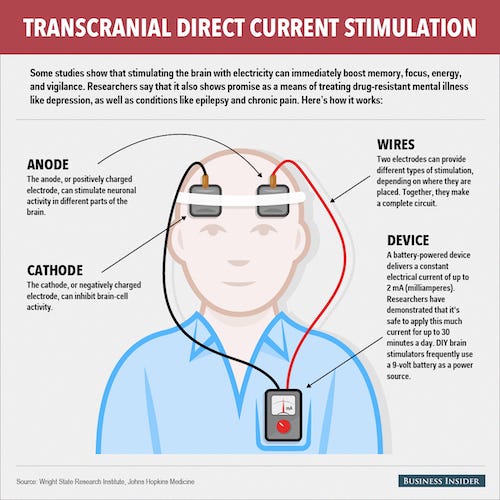
The device is to be used at home but requires a prescription from a doctor. The treatment may be used on its own or in combination with other therapies. Doctors can monitor patients’ progress and adjust treatments through a digital platform.
Sooma’s device is currently seeking FDA approval in the United States. If approved, it could help bridge the mental health gap by providing a more effective treatment for depression. Sooma has also received European Medical Device Regulation certification, making it the first tDCS device manufacturer to do so.
t/f Summary: What’s Next
The company recently secured €5 million in funding to further develop its product and expand into new and existing markets. This funding will support fast-tracking the FDA certification process, ultimately making the treatment more accessible to patients worldwide.
Sooma’s approach to treating depression offers hope to the underserved or those having limited access to traditional treatment methods. The recent funding and device designations are significant steps toward making this treatment widely available.