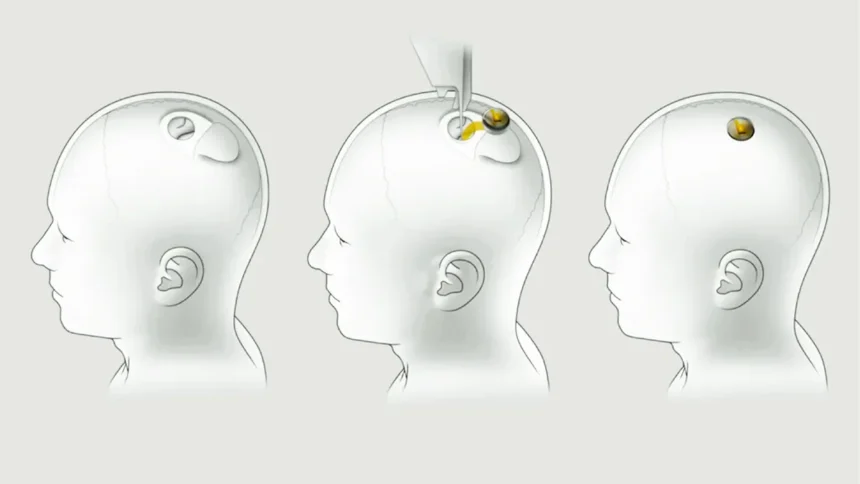
Neuralink, Elon Musk’s pioneering brain chip implant startup, recently encountered and addressed an issue with its first human implant. Neuralink is researching and developing brain-computer interface (BCI) technology. An implant, especially in the human brain, is the most delicate bio-tech integration. Neuralink’s implant is particularly noteworthy as it tests the resilience and adaptability of such advanced medical technology in real-world applications.
What’s Happening & Why This Matters
Shortly after implantation, the device installed in Neuralink’s first human subject, Noland Arbaugh, experienced an unexpected complication. Several of the chip’s connective threads retracted from Arbaugh’s brain which compromised the device’s data transmission speeds and overall effectiveness. Neuralink quickly addressed the issue by enhancing the sensitivity of the implant. The adjustments compensated for the connectivity loss and improved its performance. This quick solution demonstrates Neuralink’s readiness to tackle unforeseen challenges and ensure the functionality of their technology.
Arbaugh is part of Neuralink’s PRIME Study that evaluates the safety of the implant and the surgical robot used for its installation. The trials’ goals include enabling individuals to control digital interfaces (i.e.,g computer cursors, keyboards) purely through thought. Neuralink’s broader ambition is developing implants that could allow individuals with paralysis to control electronic devices and potentially help blind people to regain sight. These applications demonstrate the profound potential impact of integrating human cognition directly with computers.
Impact
BCI technology has both great potential and daunting challenges. Successfully overcoming these hurdles can accelerate the development of similar technologies and increase their reliability and safety. As Neuralink presses on, the need for extensive regulatory approval remains clear. The presentation and resolution of this issue will likely be studied internally and externally and could be the basis for new emergent medical technologies regulatory processes and precedents.
TF Summary: What’s Next
The successful troubleshooting of the initial problem with Neuralink’s brain implant is an important milestone towards functional, reliable brain-computer interfaces. In the future, development will continue to focus on refining the technology to ensure its safety through rigorous testing and navigating complex regulatory safeguards. As Neuralink continues to expand BCI’s boundaries, more scrutiny can be expected for med-tech engineering as it enters the next phases of innovation that could potentially transform human lives.