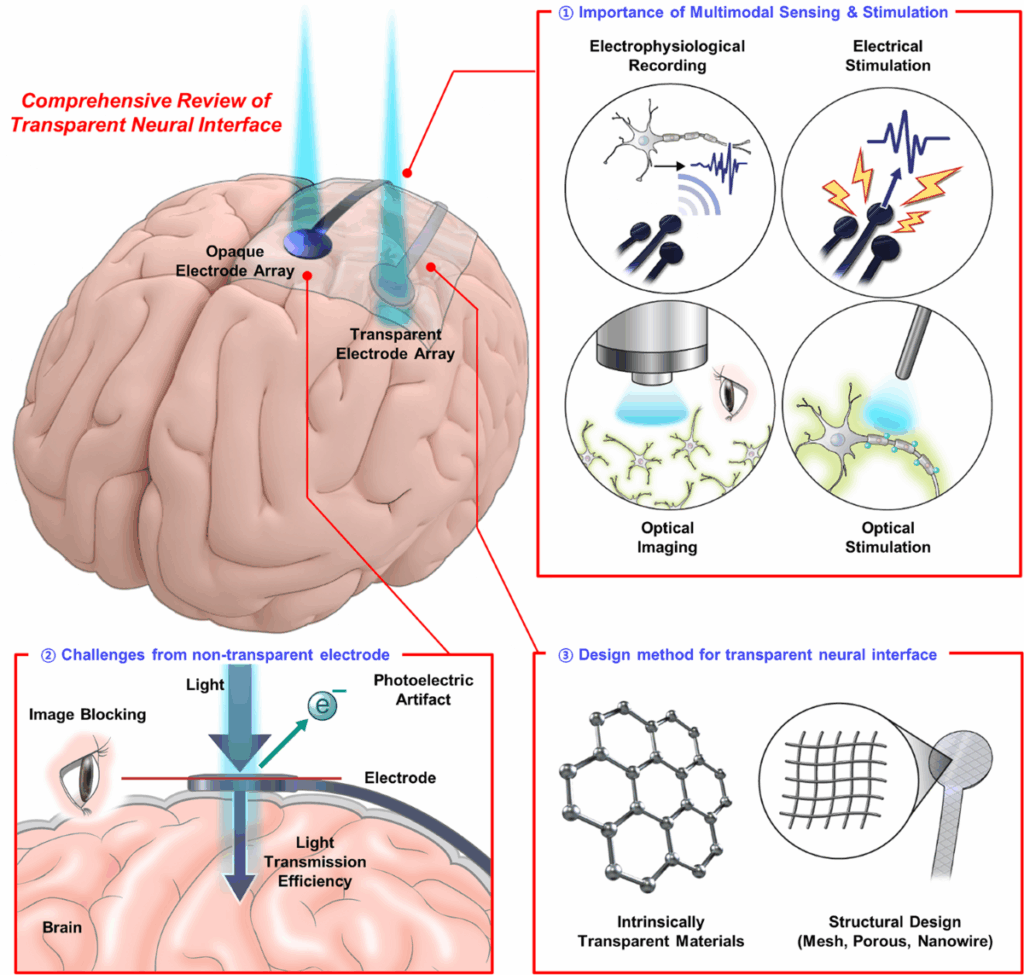
Neuralink Patients Add Control Beyond Digital Devices
Neuralink’s brain-computer interface (BCI) achieved a new feat this year. The device, once crafted as a tool for cursor control or thought-based typing, crossed into the physical world. The med-tech company shared video evidence of a trial participant controlling a robotic arm purely through neural signals. The milestone followed earlier demonstrations in which patients navigated apps, games, and keyboards via the implant.
The trial program expanded since early 2024. Neuralink implanted its chip in twelve participants with paralysis or severe neuromuscular impairment. The goal centred on restoring agency for people who lost mobility. Early volunteers included people with spinal cord injuries and amyotrophic lateral sclerosis (ALS). The newest updates broaden the project’s scope, suggesting applications far beyond screens.
What’s Happening & Why This Matters
The latest demonstration surfaced on X, another Musk-owned platform. In the clip, patient Rocky Stoutenburgh, paralysed since 2006, guides a robotic arm toward his face and kisses it. Neuralink stated that participants “extended digital computer control to physical devices such as assistive robotic arms.”
The device interprets neural activity from the motor cortex, converts it into commands, and transmits signals to paired robotics. This transition from abstract digital control to embodied physical action marked a breakthrough for the program.
Neuralink promoted the idea that the range of controllable devices increases as signal decoding improves. Stoutenburgh’s demonstration showed stable control without external joysticks, voice commands, or switches. For people with total paralysis, this marks a potential return to direct interaction with their environment.
Trial and User Registry Boom
The company disclosed that more than 10,000 people registered their interest in joining the upcoming clinical trials. Neuralink implanted twelve devices between January and September 2024. The first recipient regained the ability to play chess and video games. Others engaged with smartphones and laptops through on-screen interfaces.
Current participants include individuals with spinal cord injuries, ALS, and related mobility-limiting conditions.
The device is in early clinical testing. Researchers examine long-term durability, safety, and reliability of implanted electrodes. Regulators review engineering improvements, including wireless data transfer, low-latency decoding, and fail-safe shutdown.
BCI Competition
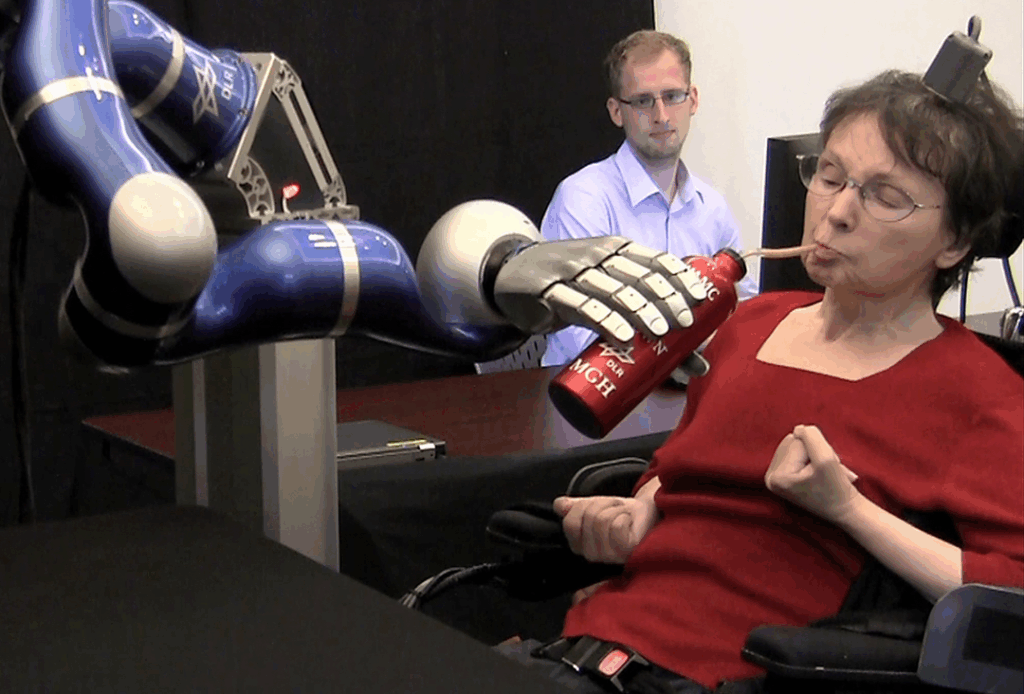
Neuralink is not alone. Multiple research groups and commercial labs explore brain-computer interfaces for paralysis, stroke recovery, cerebral palsy, and dementia. Clinical trial registries show studies investigating memory support, speech restoration, and neural rehabilitation.
Yet Neuralink stands out due to its surgical robot, wireless implant design, and public visibility through Musk.
Researchers across the field view the robotic-arm demonstration as an early confirmation that consumer-facing assistive robotics might progress from theory into practice sooner than many expected.
TF Summary: What’s Next
Neuralink’s newest demonstration comes at a moment when assistive technology is gaining momentum. BCIs enter clinics, not just labs. Stoutenburgh’s robotic-arm control is exciting progress toward functional independence for patients with paralysis. Researchers are tracking durability, precision, and user satisfaction as capabilities expand.
MY FORECAST: Neuralink upgrades its implant within two generations. Trial participants gain smoother multi-axis arm control. Partners introduce commercial assistive devices tuned for BCI integration. Insurance groups begin reviewing the technology for long-term reimbursement. Other labs accelerate competitive research, creating a fast-moving market around neural prosthetics.
— Text-to-Speech (TTS) provided by gspeech


