Apple has secured FDA approval for two new health features: a sleep apnea detection function on the Apple Watch and a hearing aid capability for the AirPods Pro 2. These advancements aim to make health monitoring and hearing assistance more accessible to the public through Apple’s widely-used devices.
What’s Happening & Why This Matters
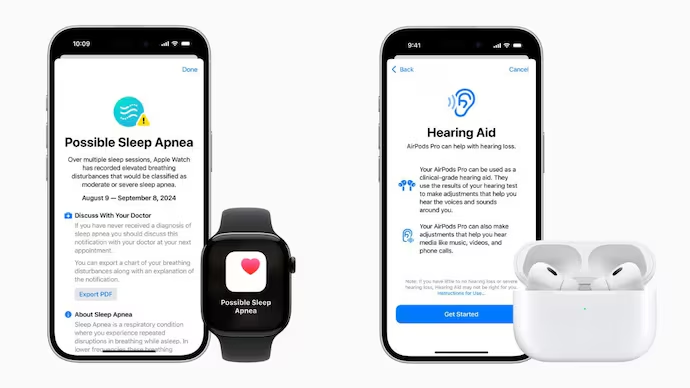
Apple Watch Sleep Apnea Detection
The FDA has greenlit Apple’s sleep apnea detection feature for the Apple Watch Series 9, Series 10, and Ultra 2 models. The feature, announced at the recent Glowtime event, uses the watch’s accelerometer to track breathing interruptions during sleep. Sleep apnea, a condition affecting over a billion people worldwide, can lead to serious health problems like hypertension, type 2 diabetes, and heart issues if left untreated.

The sleep apnea detection function logs breathing data in a new “Breathing Disturbances” section within the Health app. The data is updated every 30 days, and the watch will alert users if it detects moderate to severe sleep apnea, suggesting they consult a healthcare provider for further diagnosis and treatment. The feature is now available in more than 150 countries, including the US, EU, and Japan. Apple Watch users can access it by upgrading to watchOS 11, with Series 10 models launching on September 20.
AirPods Pro 2 Hearing Aid Capability
Apple also received FDA approval for a software update that enables AirPods Pro 2 to function as over-the-counter hearing aids. This feature was introduced at the same Glowtime event. Designed for people with mild to moderate hearing loss, the software-based hearing aid offers a more affordable alternative to traditional hearing aids, which can cost over $2,000. In contrast, AirPods Pro 2 retail at $249.
The FDA’s approval followed a clinical study involving 118 participants with mild to moderate hearing loss. The results showed that users experienced similar benefits when self-fitting the AirPods compared to a professional fitting. The study also indicated no adverse events and demonstrated comparable performance in terms of amplification and speech understanding in noisy environments.

Michelle Tarver, acting director of the FDA’s Center for Devices and Radiological Health, remarked, “Today’s marketing authorization of an over-the-counter hearing aid software on a widely used consumer audio product is another step that advances the availability, accessibility, and acceptability of hearing support for adults with perceived mild to moderate hearing loss.” Apple plans to roll out this feature in over 100 markets, including the US, later this fall.
TF Summary: What’s Next
With these FDA approvals, Apple continues to expand its wellness presence. The sleep apnea detection and hearing aid features add valuable health functions to the Apple Watch and AirPods Pro 2, making them more than just gadgets but essential tools for everyday health management. As these features become available globally, they will likely set new standards for consumer health technology, encouraging other tech companies to follow suit. The rollout of these capabilities is expected to occur throughout the fall, with potential updates and enhancements to come as Apple gathers user feedback and refines its health tech offerings.